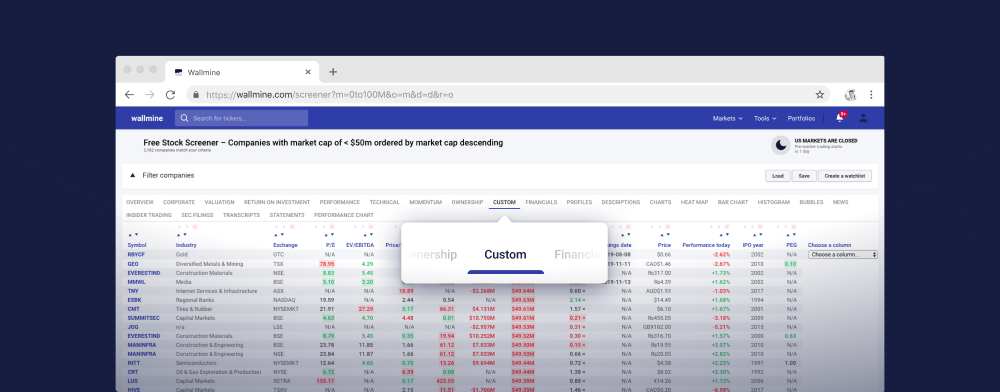
Akciový screener zdarma
Load Uložit Seznam sledování Help
Sleep Country Canada Holdings Inc. engages in retailing mattress and bedding-related products in Canada. The company offers a range of mattresses, adjustable lifestyle bases, pillows, duvets, duvet covers, mattress toppers and protectors, pet beds, weighted blankets, throws, sheets, headboards, footboards, frames, mattress and pillowcases, platforms, metal frames, blankets, mattress pads, and other sleep accessories. The company operates under the Dormez-vous, Sleep Country Canada, and Endy retail banners. As of May 20, 2022, it operated 287 stores. It also sells its products through an e-commerce platform. Sleep Country Canada Holdings Inc. was founded in 1994 and is headquartered in Brampton, Canada.
- Sleep Country Canada Holdings, Inc., 7920 Airport Road, Brampton L6T 4N8, Canada
- 289 748 0206
- sleepcountry.ca
- Investor relations

zynex, inc. founded in 1996, engineers, manufactures and markets neuro-diagnostics, stroke rehabilitation, pain management and cardiac monitoring devices within three subsidiaries. zynex medical is a provider of electrotherapy products for home use. zynex monitoring solutions develops products for cardiac monitoring for use in hospitals. zynex neurodiagnostics develops devices for emg and eeg diagnostic applications within neurology disciplines. zynex medical's product line is fully developed, fda/ce cleared, commercially sold, and has been developed to uphold the company's mission of improving the quality of life for patients suffering from impaired mobility due to stroke, spinal cord injury, or debilitating and chronic pain. zynex monitoring solutions and zynex neurodiagnostics are currently both in the development stages without any significant revenues.
- Zynex Inc, 9655 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynexmed.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. It offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) device; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. The company also supplies electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products; and distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, and LSO Back Braces for lumbar support. In addition, it offers CM-1500, a blood volume monitor device for monitoring central blood volume for use in operating and recovery rooms to detect blood loss during surgery and internal bleeding during recovery. The company provides its products for use in pain management and control; and stroke and spinal cord injury rehabilitation. Zynex, Inc. sells its products through direct sales force primarily in the United States. The company was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9555 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. It offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) device; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. The company also supplies electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products; and distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, and LSO Back Braces for lumbar support. In addition, it offers CM-1500, a blood volume monitor device for monitoring central blood volume for use in operating and recovery rooms to detect blood loss during surgery and internal bleeding during recovery. The company provides its products for use in pain management and control; and stroke and spinal cord injury rehabilitation. Zynex, Inc. sells its products through direct sales force primarily in the United States. The company was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9555 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. The company offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation, and neuromuscular electrical stimulation (NMES) device that is marketed to physicians and therapists by field sales representatives; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. It also supplies private labeled products, including electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products. In addition, the company distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, LSO Back Braces for lumbar support, and knee braces for knee support. Further, it offers Zynex Fluid Monitoring System (CM-1500), a fluid volume monitor, which is a non-invasive medical device for monitoring relative fluid volume changes used in operating and recovery rooms to detect fluid loss during surgery and internal bleeding during recovery; Zynex Wireless Fluid Monitoring System (CM-1600), a noninvasive monitoring device designed to measure relative changes in fluid volume in adult patients; NiCO CO-Oximeter, a laser-based noninvasive co-oximeter; and HemeOx tHb Oximeter, a laser-based total hemoglobin pulse oximeter. The company provides its products for use in pain management and control; stroke and spinal cord injury rehabilitation; hemodynamic monitoring; and pulse oximetry monitoring. It sells its products through direct sales force primarily in the United States. Zynex, Inc. was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9655 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. It offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) device; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. The company also supplies electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products; and distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, and LSO Back Braces for lumbar support. In addition, it offers CM-1500, a blood volume monitor device for monitoring central blood volume for use in operating and recovery rooms to detect blood loss during surgery and internal bleeding during recovery. The company provides its products for use in pain management and control; and stroke and spinal cord injury rehabilitation. Zynex, Inc. sells its products through direct sales force primarily in the United States. The company was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9555 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. The company offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation, and neuromuscular electrical stimulation (NMES) device that is marketed to physicians and therapists by field sales representatives; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. It also supplies private labeled products, including electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products. In addition, the company distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, LSO Back Braces for lumbar support, and knee braces for knee support. Further, it offers Zynex Fluid Monitoring System (CM-1500), a fluid volume monitor, which is a non-invasive medical device for monitoring relative fluid volume changes used in operating and recovery rooms to detect fluid loss during surgery and internal bleeding during recovery; Zynex Wireless Fluid Monitoring System (CM-1600), a noninvasive monitoring device designed to measure relative changes in fluid volume in adult patients; NiCO CO-Oximeter, a laser-based noninvasive co-oximeter; and HemeOx tHb Oximeter, a laser-based total hemoglobin pulse oximeter. The company provides its products for use in pain management and control; stroke and spinal cord injury rehabilitation; hemodynamic monitoring; and pulse oximetry monitoring. It sells its products through direct sales force primarily in the United States. Zynex, Inc. was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9655 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. It offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) device; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. The company also supplies electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products; and distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, and LSO Back Braces for lumbar support. In addition, it offers CM-1500, a blood volume monitor device for monitoring central blood volume for use in operating and recovery rooms to detect blood loss during surgery and internal bleeding during recovery. The company provides its products for use in pain management and control; and stroke and spinal cord injury rehabilitation. Zynex, Inc. sells its products through direct sales force primarily in the United States. The company was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9555 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations
ZYXI

Zynex, Inc., through its subsidiaries, designs, manufactures, and markets medical devices to treat chronic and acute pain; and activate and exercise muscles for rehabilitative purposes with electrical stimulation. It offers NexWave, a dual channel, multi-modality interferential current, transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) device; NeuroMove, an electromyography triggered electrical stimulation device; InWave, an electrical stimulation product for the treatment of female urinary incontinence; and E-Wave, an NMES device. The company also supplies electrodes for the delivery of electrical current to the body, and batteries for use in electrotherapy products; and distributes Comfortrac/Saunders for cervical traction, JetStream for hot/cold therapy, and LSO Back Braces for lumbar support. In addition, it offers CM-1500, a blood volume monitor device for monitoring central blood volume for use in operating and recovery rooms to detect blood loss during surgery and internal bleeding during recovery. The company provides its products for use in pain management and control; and stroke and spinal cord injury rehabilitation. Zynex, Inc. sells its products through direct sales force primarily in the United States. The company was founded in 1996 and is headquartered in Englewood, Colorado.
- Zynex, Inc., 9555 Maroon Circle, Englewood 80112, United States
- 303 703 4906
- zynex.com
- Investor relations